Men on sodium valproate told to use contraception
There is a potential, small increased risk of autism and other neurodevelopmental problems, experts say
 Getty Images
Getty ImagesMen taking sodium valproate are being warned to use contraception while on the medicine, because of a “potential small increased risk” of autism and other neurodevelopmental problems for any children conceived.
They should continue to do so – and cannot donate sperm – until three months after they have stopped taking the drug.
Sodium valproate, prescribed under brand names including Epilim, Belvo, Convulex and Depakote, is an effective treatment for epilepsy and bipolar disorder.
The Medicines and Healthcare products Regulatory Agency (MHRA), which issued the warning, stressed patients must speak to their doctors before making any changes to their medicines.
‘Safety issue’
The guidance follows a similar warning from the European Medicines Agency, after data from national registries in Norway, Denmark and Sweden suggested 5% of children born to men taking the drug were harmed.
That study did not prove sodium valproate was the cause, the MHRA said, or compare risks for children whose fathers were not on medication.
But it raised an “important safety issue that warrants action on a precautionary basis”.
Sodium valproate was already known to make it more difficult to father a child.
But the MHRA says this is usually reversible after the drug is stopped.
Chief Executive of the Epilepsy Society, Clare Pelham welcomed the move by the MHRA but said they could have acted sooner.
“It is right for the MHRA to be vigilant as more information becomes available about the risks of epilepsy medication; and to bring this to public attention. They have not always done so. People with epilepsy must be able to make informed choices about the drugs they wish to take.”
Babies exposed to sodium valproate in the womb have a 40% risk of of autism and other neurodevelopmental problems and a 10% risk of physical abnormalities.
An estimated 20,000 children in the UK have had life-changing injuries from the medicine.
Families in the UK and abroad say their concerns about the effects of the drug on their children were dismissed for years before the European Medicines Agency issued warnings in 2018.
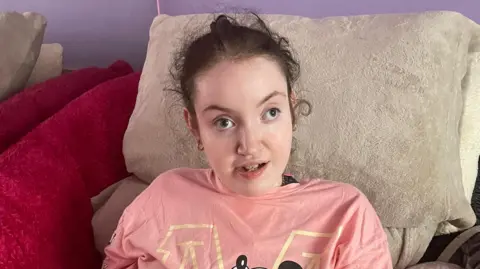
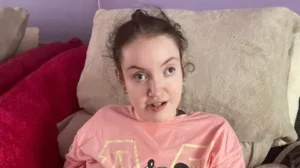
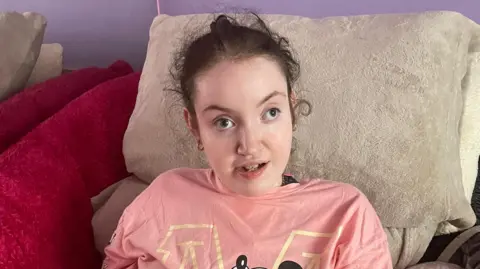
The group OACS which supports families affected by Valproate drugs has criticised the MHRA for failing to investigate safety issues regarding fathers sooner.
Spokesperson Karen Buck took the drug for her epilepsy while pregnant.
Her daughter Bridget, who is now 26 years old, has Fetal Valproate Syndrome and is bed bound, has severe brain damage and requires 24-hour care.
Karen says she asked the regulator to include the issue at a meeting in 2016 but was told it would only examine problems relating to women.
“This is shameless. This should have went out when I told them way way back. And I said to the MHRA and I sat there with all my colleagues from the charities. You need to put the word out; not just for women, for men as well. I said to them, I told you from day dot that men are being affected.”
And in January, the MHRA warned under-55s should not take it unless all other treatment options had been rejected and its use had been signed off by two independent specialists.
Nevertheless, 65,000 children and adults under the age of 55 still take the sodium valproate.
MHRA chief safety officer Dr Alison Cave said: “Patients on valproate should not stop taking their medicine unless advised to do so by a healthcare professional.
“It is important to attend your next appointment in order to discuss your treatment plan.”